在6月2日的胃肠道(大肠)癌口头报告上,有报告称,与单纯5-FU/LV相比,FOLFOX辅助治疗----一种奥沙利铂,5 -氟尿嘧啶(5-FU)和亚叶酸(LV)治疗方案,可显著提高术前放化疗且手术治疗后的ypStageII 期(新辅助治疗后病理分期II)或ypStageIII期直肠癌患者的3年无病生存率(DFS)(摘要3502)。来自韩国蔚山大学医学院,报告ADORE研究的Tae Won Kim博士说:“这是显示FOLFOX方案对术前放化疗和手术后直肠癌患者有临床疗效的首个随机研究。”
虽然基于奥沙利铂的辅助性治疗是大肠癌患者术后的标准疗法,但其在II/III期直肠癌中使用的相关信息仅来源于在结肠癌治疗机构进行的研究。由于缺乏支持广泛使用这种疗法的数据,ADORE研究是一项随机II期研究,对术前放化疗并进行根治性手术后患者进行辅助性FOLFOX方案(160例)或5-FU/LV(161例)治疗。FOLFOX方案每2周一次,为期8个疗程;5-FU/LV每4周一次,为期4个疗程。
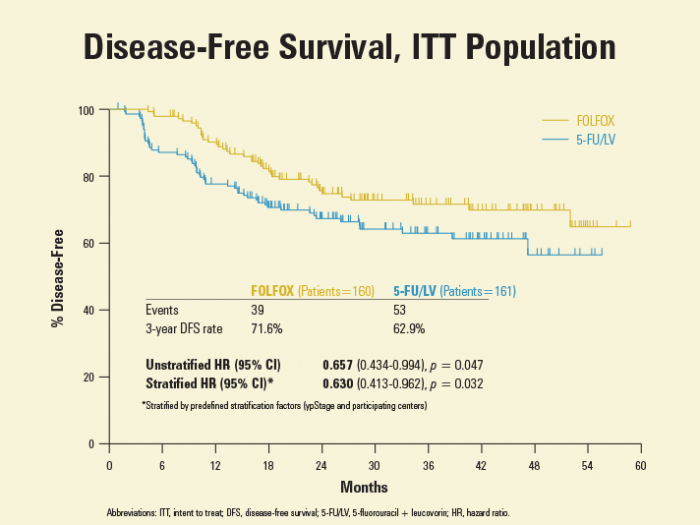
38.2个月的中位随访显示,辅助性FOLFOX组及辅助性5-FU/LV组的3年DFS率分别为71.6%,62.9%。0.66的粗分风险比(HR)(95%CI [0.434-0.99,P = 0.047)下,5-FU/LV中加入奥沙利铂可增加34%的3年无病率。据Kim博士称,这些数据与那些结肠癌患者报告数据类似,FOLFOX方案与结肠癌患者使用的一致。甚至在调整了预定义的分层因素(ypStage分期和参与的医疗中心)后,分层HR为0.63(95%CI [0.413-0.962],P = 0.032),数据仍有利于辅助FOLFOX治疗患者(图1)。ypStage III期患者3年DFS优势较明显,但不适用于那些ypStage II期直肠癌患者。
佛罗里达大学的Carmen Joseph Allegra博士,对评估奥沙利铂辅助性治疗直肠癌的三项研究进行了讨论,并比较了他们的研究设计。与其他两项研究(摘要3500和摘要3501)不同,ADORE研究对接受新辅助治疗及手术治疗后的患者进行了随机分组。Allegra 博士说,ADORE研究人员确保了指定辅助治疗的患者大多数进行了辅助治疗。
Allegra 博士讨论了“新辅助时代”直肠辅助治疗的作用,并评论说:“全部证据强烈支持辅助治疗对直肠癌患者有益。”他接着讨论了辅助性奥沙利铂对直肠癌的作用,这提示奥沙利铂可能有临床疗效。根据MOSAIC和NSABP C-07试验显示,辅助性5-FU/LV中添加辅助性奥沙利铂对局限性晚期结肠癌有益,他说。他进一步提出,直肠和结肠癌类似的遗传基础可能意味着类似的治疗方案。
Allegra博士告诉ASCO每日新闻称,ADORE研究人群经高度选择,因为如果患者出现完全病理学应答或新辅助治疗后分期降至I期,则患者不符合研究标准。他补充说,“我们不能说每一例开始新辅助治疗和手术的患者将受益于辅助性FOLFOX治疗。然而,对于我们使用FOLFOX方案治疗的医生来说,ADORE确定了FOLFOX治疗在辅助性直肠癌治疗中的价值。”
编译自:Adjuvant FOLFOX after Neoadjuvant Chemoradiotherapy Reported to Significantly Improve DFS in Patients with Rectal Cancer.ASCO DAILY NEWS,June 3,2014.
医脉通整理报道,转载请注明出处。
会议专题》》》2014年ASCO年会专题报道
阅读英文摘要
Adjuvant chemotherapy with oxaliplatin/5-fluorouracil/leucovorin (FOLFOX) versus 5-fluorouracil/leucovorin (FL) for rectal cancer patients whose postoperative yp stage 2 or 3 after preoperative chemoradiotherapy: Updated results of 3-year disease-free survival from a randomized phase II study (The ADORE).(Abstract No:3502)
Authors:Yong Sang Hong, Byung-Ho Nam, Tae-You Kim, et al.
Session Type: Oral Abstract Session
Background: To report the updated results of 5-fluorouracil (5-FU)/leucovorin (LV) (FL) with or without oxaliplatin (FOLFOX) in patients with curatively resected rectal cancer whose pathologic stages of ypII/III after preoperative chemoradiotherapy (CRT).
Methods: This is a randomised phase II study accrued patients with curatively resected rectal cancer patients whose postoperative stage ypII (ypT3-4/ypN0) or III (any ypT/ypN1-2) after preoperative CRT with fluoropyrimidines alone. Patients were randomly assigned (1:1) to receive adjuvant chemotherapy either with FL (5-FU 380 mg/m2, LV 20 mg/m2 on days 1-5, every 4 weeks, 4 cycles) or FOLFOX (oxaliplatin 85 mg/m2, LV 200 mg/m2, 5-FU bolus 400 mg/m2 on day 1, 5-FU infusion 2400 mg/m2 for 46 hours, every 2 weeks, 8 cycles). Randomisation was centrally coordinated and stratified by the ypStage and participating sites. The primary endpoint was 3-year disease-free survival (DFS).
Results: A total of 321 patients were randomly assigned between November 2008 and June 2012; 161 patients to FL and 160 to FOLFOX. The arms were balanced. At a median follow-up of 38.2 months (IQR, 26.4 – 50.6), 3-year DFS rate was 71.6% (95% CI, 64.6 – 78.6) in the FOLFOX arm and 62.9% (95% CI, 55.4 – 70.4) in the FL arm with a hazard ratio (HR) of 0.657 (95% CI, 0.434 – 0.994, p=0.047) by intention-to-treat analysis. After adjusting for stratification and prognostic variables, HR remained unchanged favoring FOLFOX (0.560; 95% CI 0.366 – 0.856; p=0.007) in terms of 3-year DFS. In the subgroup analysis, patients with ypStage III (HR 0.602 [0.371 – 0.977], p=0.040), ypN1b (HR 0.356 [0.132 – 0.960], p=0.041), ypN2 (HR 0.414 [0.181 – 0.946], p=0.037), and minimally regressed tumors (HR 0.395 [0.188 – 0.831, p=0.014] benefited more from FOLFOX than FL. Grade-3 or -4 adverse events were not statistically different between arms.
Conclusions: Adjuvant FOLFOX demonstrated improved 3-year DFS in curatively resected rectal cancer patients whose postoperative stage of ypII/III after preoperative CRT. Clinical trial information: NCT00807911.
